8 February, 2018
Following our previous Client Alert from June 2017 on 31 January 2018 the Department of Intellectual Property (the DIP) published the second draft amendment to the Thai Patent Act (the Draft) on its website and opened a public hearing to run from today through 28 February 2018. In addition to the Draft, the DIP also published several supplementary documents and flowcharts regarding the amendments. The Draft and the documents are only available in Thai.
The key amendments are as follows:
Non-Patentable Subject Matter
“Business methods” are added to this Draft as a non-patentable subject matter. The rationale mentioned in the Draft is that business methods do not fall under the Thai Patent Act’s definition of an invention. We believe that there will be concerns raised whether this exception contravenes the “Thailand 4.0” digital economic model, as said model aims to drive the country through the implementation of digitized innovations.
It is worth noting that as discussed in our Client Alert in June 2017, surgical methods will also become a non-patentable subject matter. The term “surgical methods” has been added to comply with TRIPS Article 27(3), which seems to encompass both therapeutic and non-therapeutic treatments, such as cosmetic treatments.
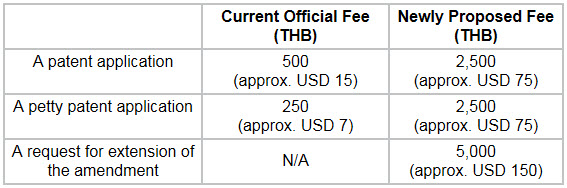
Official Fees
There has been a substantial increase in the newly proposed official fees which were published in the Draft. These changes are highlighted below.
Please click on the table to enlarge.
Access to Genetic Resources and Benefit Sharing
The obligation to declare the source of genetic resources or traditional knowledge, and submit documents concerning “prior informed consent” (PIC) and “mutually agreed terms” (MAT), has been maintained in this Draft. The DIP's supplementary document, however, has clarified that the obligation to obtain PIC and set up MAT will not apply to genetic resources derived from microorganisms and animals collected in Thailand. Furthermore, the obligation will not apply to genetic resources collected in a country that has no laws regarding the recognition of genetic resources and traditional knowledge, nor that taken from places outside any sovereignty’s border, such as the high seas, and for information disclosed on the internet. Please note that in the former case, the patent applicant still has the obligation to state the country of origin and to warrant that there are no laws regarding the recognition of genetic resources and traditional knowledge in said country. For cases of the latter type, the patent applicant must provide the geographical information or cite an internet reference from which said genetic resources or traditional knowledge has been taken.
It is also worth noting that the abovementioned supplementary document also provides further information on the patent applicant’s course of action in respect of said obligation prior to, during, and after the filing of a Thai patent application.
We will continue to closely monitor the progress of the Draft and provide updates on developments as they occur.
For further information, please contact:
Say Sujintaya, Partner, Baker & McKenzie
say.sujintaya@bakermckenzie.com