17 August, 2018
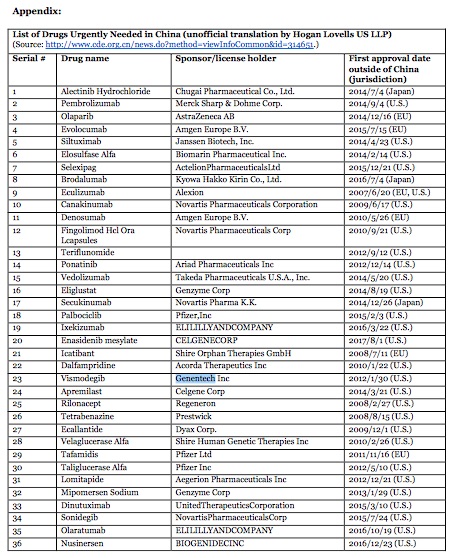
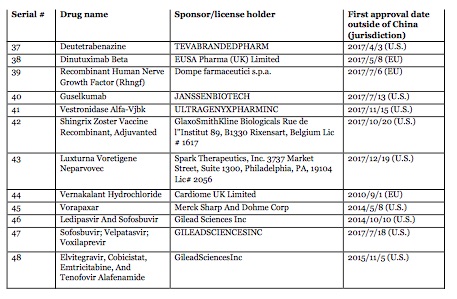
On 8 August 2018, in an unprecedented regulatory action, China National Drug Administration (CNDA) (formerly known as CFDA) called on international pharmaceutical companies to bring 48 new drugs to China. These new drugs are viewed to be urgently needed in China and have already been approved and marketed in the U.S., EU and Japan for rare diseases or other life-threatening diseases and conditions. CNDA stated that to the extent the drug sponsors can submit data demonstrating there are no racial or ethnic differences that would affect the efficacy, they are encouraged to submit their new drug applications now without conducting clinical trials in China. Once the drug applications are submitted, the CNDA will prioritize their review. The drug sponsors are also encouraged to schedule meetings with the Center for Drug Evaluation of CNDA if they have any application-related questions.
CNDA’s data requirements for the new drug applications can be summarized as follows:
- Proof of approval in U.S., EU, or Japan by corresponding drug regulators; proof of marketing in Japan, Hong Kong, Macau, or Taiwan and export records to these areas for the past five years.
- Application data package meeting the CTD requirements. Certain key documents should be in Chinese.
- Racial sensitivity analysis report based on relevant guidance documents from ICH.
- Post-market research and risk control plan.
- Statement of consistency.
Public comments for the list and new drug application data requirements are due on 18 August 2018. It is unclear at this point whether CNDA will consider additional new drugs to be added to the list. It is also unclear what data CNDA requires to demonstrate no racial or ethnic differences that would affect the efficacy. Regardless, this is yet another encouraging development in China for international pharmaceutical companies.
We will continue to monitor the status of any regulatory changes and reforms by CNDA. With offices in Washington DC and Beijing, and a team of life science attorneys well versed in both FDA and CNDA regulations, Hogan Lovells is well-positioned to assist international pharmaceutical companies in launching their innovative drug products in China. Please contact one of the authors of this alert if you have any questions or if we can be of further assistance.
Please click on the tables to enlarge.