13 April, 2020
On March 5, 2020, the National Medical Products Administration (“NMPA”) promulgated the Announcement on the Transfer of Medical Devices with Imported Registration Certificates to Chinese Domestic Enterprises for Production (Draft for Comment) (“Draft Notice”) (“《已获进口医疗器械注册证的产品转移中国境内企业生产有关事项公告(征求意见稿)》” in Chinese). The Draft Notice, once adopted, will provide an expedited pathway for the localization of imported medical devices and IVD products. Here is our summary and analysis of the Draft Notice.
I. Background
The Opinions on Deepening the Reform of the Evaluation and Approval Systems and Encouraging Innovation of Medicine and Medical Devices (“《关于深化审评审批制度改革鼓励药品药疗器械创新的意见》” in Chinese) promulgated by the State Council on October 8, 2017 introduced the Marketing Authorization Holder (MAH) system for devices. By the end of 2019, 21 provinces in China had introduced trial MAH programs for devices, which allows the separation of the MAH holder and the manufacturer of medical devices. However, imported devices were not included in these trial programs. In other words, a foreign company which holds the registration certificate for an imported device must manufacture the device outside China. If a foreign company intends to manufacture the device in China, it must license or transfer the product and technology to a Chinese company. The Chinese company then needs to complete the product registration or record-filing of the same device as a domestic device, going through a time-consuming and somewhat repetitive process of registration, testing, clinical evaluation and document submission required by the NMPA, with exemptions only for Class I devices and some products exempted for clinical evaluation.
II. The Draft Notice
According to the Draft Notice, a foreign holder of a registration certificate for imported Class II/III devices or IVD products (“Foreign Holder”) may have its affiliate in China (“Domestic Holder”) register the same product as a domestic product and hold the relevant registration certificate. The NMPA allows the Domestic Holder to use certain application materials lodged during the registration of an imported product, to accelerate the registration process. The specific requirements are summarized as follows:
Applicable Products
Class II and Class III medical devices/IVD products already registered with the NMPA
Qualification for Domestic Holders
1. A foreign-invested company in China whose majority interest is owned by the Foreign Holder
2. Where the Foreign Holder is controlled by a Chinese company, the Chinese company can be the Domestic Holder.
Registration Materials
1. Applicants shall submit registration materials in accordance with the NMPA requirements.
2. The following registration materials of the imported products may be used for the registration of the domestic products:
(a) Medical devices: general information, research materials, clinical evaluation materials, and product risk analysis materials;
(b) IVD products: general information, research materials on raw materials, performance testing materials, materials for positive value or scope, stability materials, clinical evaluation materials and product risk analysis materials.
3. Declaration of consistency: both the Foreign Holder and the Domestic Holder shall ensure that the aforesaid registration materials are consistent with the original registration materials for imported registration.
Inspection Requirements
1. The applicant shall provide the relevant materials on quality management systems for overseas manufacturing.
2. During on-site inspections, the NMPA shall focus on the consistency and traceability of the domestic quality management system and the overseas quality management system in the fields of design and development, procurement control, production control, quality control, etc.
3. Respective on-site inspections pertaining to manufacturing licenses and product registration may be conducted simultaneously to avoid repetition.
Post-commercialization Requirements
1. After obtaining a registration certificate, the Domestic Holder shall apply for a manufacturing license in accordance with the relevant requirements and procedures set out in the Regulations on the Supervision and Administration of the Manufacturing of Medical Devices (“《医疗器械生产监督管理办法》”in Chinese).
2. Domestic Holders shall bear the main responsibility for the quality and safety of the products and be responsible for quality management throughout their life cycles.
III. Our Observations
1. Localization of imported products fast tracked
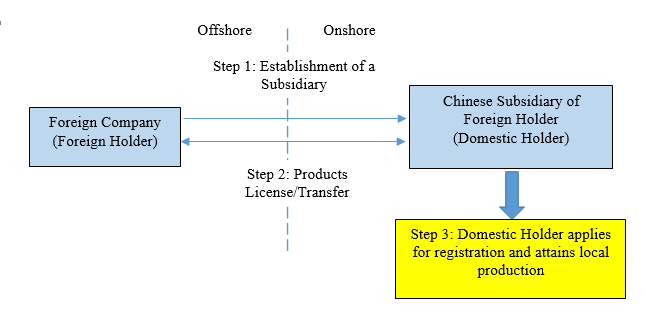
A major change introduced by this Draft Notice is that imported medical devices and IVD products which have been registered with the NMPA have now been fast tracked to be registered as domestic products and be produced in China. As mentioned above, under the existing legal regime, it was time consuming and costly to register imported products as domestic products and localize their production in China. Under the new pathway, the NMPA allows applicants to use certain application materials used for registering imported products, particularly clinical evaluation materials, during the registration of the same products as domestic products. The simplification of clinical evaluation will substantially facilitate the localization of imported medical devices. After the implementation of the Draft Notice, certain Foreign Holders may choose to license or transfer some imported products to its subsidiaries in China, which will act as the Domestic Holder to realize local production, so as to optimize its supply chain and reduce production costs. Such a business model is illustrated as follows:
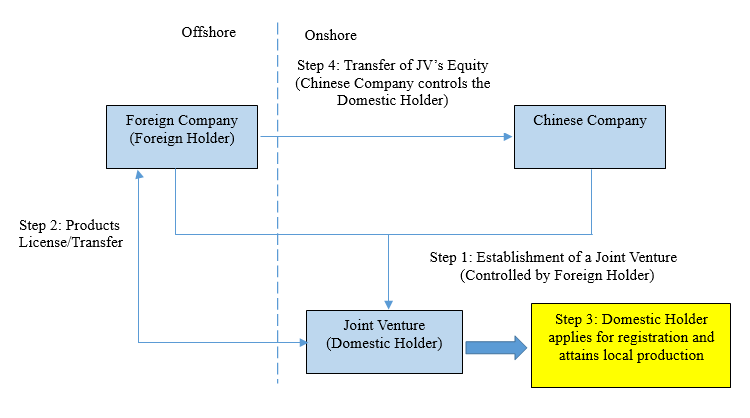
If a Foreign Holder intends to divest and sell the commercial rights of its portfolio products in China, they may also utilize the new pathway by establishing a joint venture company in China with the Chinese buyer, and then transfer the portfolio rights to the joint venture company for registration and local production. After the joint venture company becomes the Domestic Holder of the registration certificates of the portfolio products, the Foreign Holder can then sell its equity in the joint venture company to the buyer and allow the purchaser to control the joint venture company. This business model is illustrated below:
2. Domestic registration and production cannot be separated
Regrettably, the Draft Notice requires that the Domestic Holder must also be the holder of the production licenses of the relevant products. In other words, the Domestic Holder needs to have the full capability to produce the products in China. This increases the operating costs of the Domestic Holder and deviates from the original goal of the MAH system which aims to separate registration from production, as currently advocated by the NMPA. The NMPA may want to introduce the reform gradually and prevent abuse of the new law. The Draft Notice does not prohibit the Domestic Holder from engaging contract manufacturers after the domestic registration is completed.
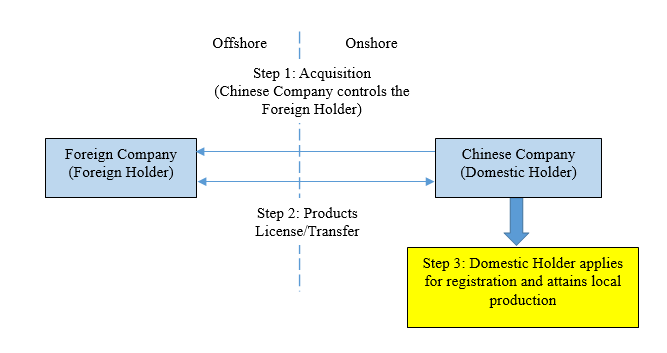
3. Implications on the acquisition of foreign device companies
The Draft Notice provides that if a Chinese company controls a Foreign Holder, the Chinese enterprise can serve as the Domestic Holder. Therefore, a Chinese company may acquire a Foreign Holder which owns an imported product portfolio already registered with the NMPA and quickly localize the portfolio through the pathways introduced by the Draft Notice. This business model is illustrated below:
4. Statutory obligations of the Foreign Holder
The Foreign Holder is required to undertake certain statutory obligations when the Domestic Holder registers the relevant products as domestic products. According to the Draft Notice, the registration materials lodged by the Domestic Holder must include the following statements given by the Foreign Holder: (1) a statement of approval or power of attorney, which grants authorization to the Domestic Holder to lodge the application, and authorizes the Domestic Holder to use the original registration materials; (2) a statement on the consistency of the quality management systems, stating that the quality management systems of the domestic product and the imported product are the same in the fields of design and development, procurement control, production control and quality control, etc.; and (3) a statement of guarantee on the truthfulness of the submitted materials. The Foreign Holder should be aware of the legal liabilities that may arise from the aforesaid statements and seek indemnity from the Domestic Holder and/or its Chinese partners in relation to losses which may arise from such statements.
IV. Conclusion
The Draft Notice is a breakthrough in accelerating the localization of imported medical devices and IVD products. It simplifies the domestic registration process of imported medical devices and reduces the costs to the applicants. After the Draft Notice is implemented, the localization of imported medical devices will be accelerated, which will encourage domestic production, increase the accessibility of medical devices to patients, and better serve public health demands.
The Draft Notice is open for public comments until March 31, 2020, after which the NMPA will make further revisions to reflect public concerns. We will continue to follow up the development of the law and keep you posted.